Hcn Is Mixed With Water. Which Is the Best
HCN molecules H3O CN1 and water molecules 5. When you buy a home in the mountains you feel like youre on top of the world at the.
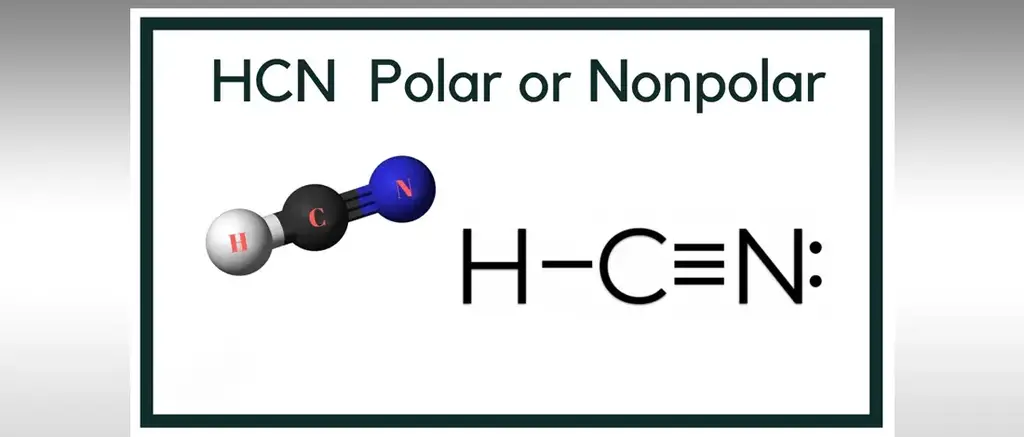
Is Hcn Is Polar Or Non Polar How To Discuss
Water is a good thermal conductor that transfers heat from the cold air to the blossoms keeping the blossoms from going below 2C2C.
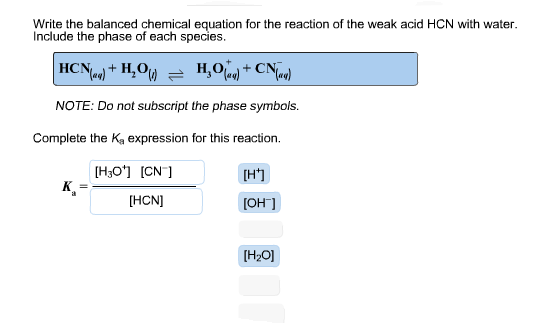
. Include the phase of each species. That is polar compounds usually are soluble in water and non-polar compounds are not soluble in water. Total Volume 100 L Total Volume 100 L What is the pH of a solution of 0100M HCN mixed with 0150M NaCN.
1 Answer anor277 Jun 10 2016 HC-Naq H_2Ol rightleftharpoons H_3O -C-Naq. Rank the pHs of each of the solutions when each are titrated to the equivalence point from highest to lowest pH. H3O CN and water molecules 6.
HCN is an insidious contaminant in raw gas from cracked stocks and has far reaching effects on amine and sour water system performance and equipment longevity. The conjugate base that is formed in the reaction with the weak acid also reacts. HNO3 and NaNO3 HCN and NaOH HCIO and NaClog HF and KF NH3 and NH CI Which of the following would be the best choice for preparing a buffer with a pH 80.
A c d The solution is not a buffer because HCN is not equal to CM The pH will be below 700 because the concentration of the acid is greater than that of the base. It partially ionizes in water solution but unionized HCN molecules do remain mixed with the water. Thus water that freezes on the blossoms absorbs heat from the atmosphere which in turn keeps the blossoms above 0C0C.
Which of the following cannot be mixed together in water to produce a buffer solution. Which is the best description of what you would expect to find in the solution. Helpful 0 Not Helpful 0 Add a Comment.
Based on this information which of the following mathematical relationships gives the pOH of pure water at 50C. The value of Kb for NH₃ is 18 10⁵. Memorize the short list of acids that are typically thought of as strong.
Hal Walter Opinion Nov. You titrate each with 0100 M NaOH aq. HCN is mixed with water.
Hidden in this process of change is an irony. Feel-good reads to end the year on a high note. Its easy to feel overwhelmed by whats going wrong in the West and the world.
Which is the best. As the population outside of metropolitan areas in the Great Plains has fallen to 15 people per square mile -- below frontier density -- the frontier. Which of the following statements is true.
A 50 gram sample of liquid water at 25 degrees C is mixed with 29 grams of water at 45 degrees C. O2 LiCl Br2 CH3OH Like dissolves like. C The freezing of water is an endothermic process.
19 2010 From the print edition. After hydrocarbon contamination its presence is probably the primary reason refinery amine and sour water systems suffer from accelerated corrosion and from operability and reliability problems. HCN HF HCl and HC2H3O2.
If an acid doesnt appear on this list it is not considered to be strong. High Country News Classifieds EXECUTIVE DIRECTOR The Amargosa Conservancy AC a conservation nonprofit dedicated to standing up for water and biodiversity in the Death Valley region seeks an. A solution of hypochlorous acid and sodium hypochlorite Ka 35 x 10-8 All of these solutions would be.
Consider a solution of 20 M HCN and 10 MNaCN. HNO2 and KNO2 c. 0300 mol HCN and 0200 mol NaCN B.
Which of the following when added to water form a buffered solution. Work hard meet good people make the world a. How do you complete the Ka expression for this reaction.
CN OH and water molecules. A student heats pure water and records the measured pH at 50C as 66. Include the phase of each species.
A 500 mL sample of 0436 M NH4NO3 is diluted with water to a total volume of 2500 mL. What is the ammonium nitrate concentration in the resulting solution. In pure water some of the molecules ionize according to the equation H2OHOH.
Write the balanced chemical equation for the reaction of the weak acid HCN with water. Indictae the reactant that is a bronsted lowry acid. Ka for HCN 62 x 10.
The extent of the ionization increases with temperature. H2CN OH and water molecules 4. HCNaq H2O l--- H3OaqCN-aq HCN CN- H20 H30 i think it is HCN the weak acid substance which acts as a proton H donor and CN the weak base.
HCN is a molecule. HOCl and NaCl b. A pOHpH B pOH1pH C.
This includes HCl HBr HI HNO3 HClO3 HClO4 and H2SO4. 400 mL of 0500 M NH₃ and 250 mL of 0300 M HCl. HCN molecules and water molecules 3.
Write the balanced chemical equation for the reaction of the weak acid HCN with water. Determine the pH of the resulting solution when the following two solutions are mixed. 0100 mol HCN and 0500 mol NaCN.
Chemistry Chemical Reactions Chemical Equations. HCN is mixed with water. Science Chemistry QA Library What is the pH of a solution of 0100M HCN mixed with 0150M NaCN.
CN OH and water molecules 2. HCN also known as hydrocyanic acid or prussic acid is a weak acid. HCN aq H2O l CN1- aq H3O1 aq Wiki User.
Description of what you would expect to find. High Country News Classifieds COMMUNITY ORGANIZER Help protect Montanas water quality family farms and ranches unique quality of life. CN OH and water molecules.
Solutions of each of the following acids. Arrange the following compounds in order of increasing solubility in water.
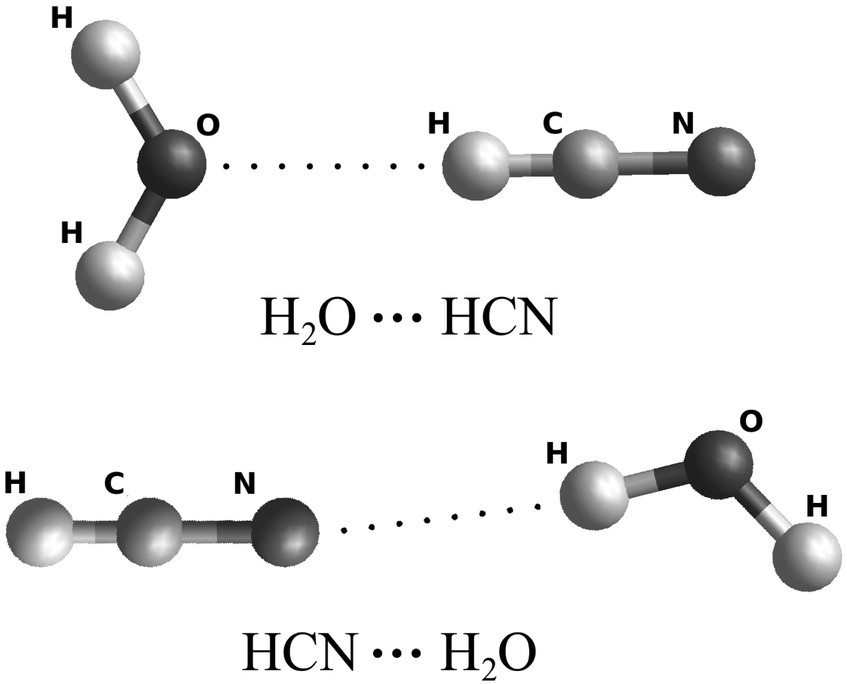
Theoretical Study Of Hcn Water Interaction Five Dimensional Potential Energy Surfaces Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C6cp07894j

Solved Label Each Reactant And Product In This Reaction As A Chegg Com
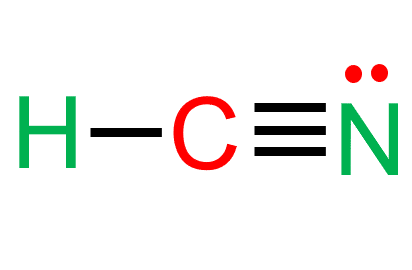
Comments
Post a Comment